Cathodic protection explained
Cathodic protection is a method to prevent corrosion in buried and submerged metallic structures. It allows extending the life of the structures, obtaining both high benefits to the safety and reliability of the installations, as well as significant reduction of total costs during the life of the assets.
It is commonly used to protect numerous structures against corrosion, such as pipelines, tanks, vessels, ships, pilings, docks, marine floats, underwater equipmentsuch as pipelines, tanks, vessels, ships, pilings, docks, marine floats, underwater equipment and a large number of other applications, basically all buried or submerged metallic structures.
Basic principles of cathodic protection
The principle is based on converting active areas of a metallic surface into passive ones, i.e. converting them into the cathode of an electrochemical cell. Learn more about corrosion here.
By supplying impressed or galvanic current, the metal potential is reduced, corrosion attack ceases and corrosion protection is achieved. Cathodic protection methods depending on the energy source used can be as follows:
Cathodic protection by sacrificial galvanic anodes
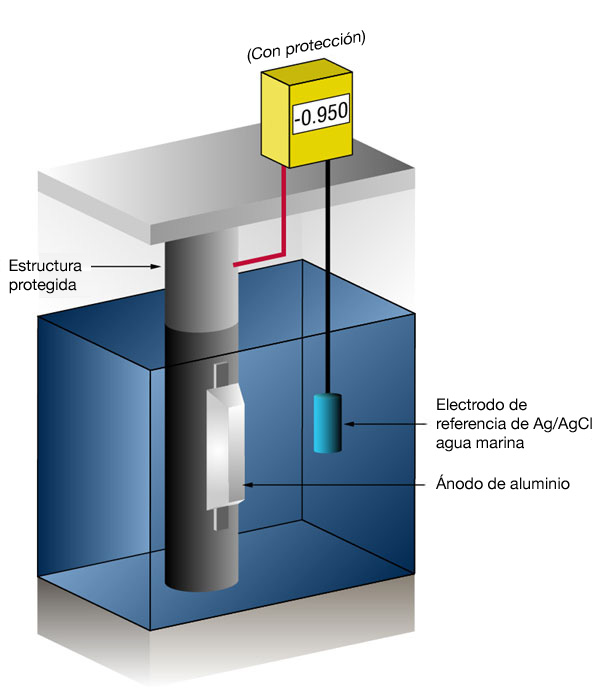
The simplest method of applying cathodic protection is to connect the metal to be protected (cathode) with another metal that corrodes more easily, so that the latter acts as an anode. Zinc, aluminum and magnesium are the metals commonly used as anodes to protect steel or iron structures.
As the activation voltage of sacrificial anodes is low compared to impressed current anodes, sacrificial anodes should be well distributed and located closer to the area being protected. It is feasible to use them when point protection is required and the budget investment is lower than that required for impressed current systems.

Impressed current cathodic protection (ICCP)
ICCP (impressed current cathodic protection) systems use electrical energy provided by an external regulated direct current power supply through a control panel. The control panel regulates the current necessary to polarize the surface to be protected.
The protective current is distributed by specially designed, inert anodes, usually employing a conductive material that does not corrode easily. One of the most common ICCP anode types for application is the “MMO (Mixed Metal Oxide) / Ti (Titanium) base” anode, which consists of a titanium (Ti) substrate coated with a metal oxide (MMO).
At Tecna ICE we have a wide experience and highly specialized personnel in cathodic protection, corrosion control and integrity of on-shore and off-shore installations.NACE (AMPP) certified.
Did you find this content useful?
Subscribe and receive technical articles, updates and more in your inbox.
Looking for a solution like this?
Explore our specialized services in integrity engineering and applied technology.